Phenotypic Screening
Phenotypic screening is the most successful screening approach in CNS drug discovery. All definitions of phenotypic screening refer to a physiological read-out as a phenotype of a complex cell system in vitro or in vivo. Phenotypes described by the electrophysiological activity of neuronal networks are ideal for phenotypic screening since they incorporate the natural physiological function of the brain.
The success of phenotypic screening approaches depends on the assumption that the respective disease mechanisms are physiologically adequately presented in the model system. For in vitro models, this requirement for relevance for disease mechanisms demands a comprehensive and sophisticated validation strategy.
NeuroProof has developed in vitro disease models for different types of CNS diseases, such as neurodegenerative, neurological, and psychiatric diseases. NeuroProof aims to develop read-outs that provide consistency with clinically translatable biomarkers.
For this, NeuroProof performs its functional phenotypic screening services with neuronal micro-circuits containing a multitude of physiologically relevant receptors with primary neuronal cell cultures or human pluripotent stem cell, iPSC-derived cultures grown on microelectrode array neurochips.
We compare the functional fingerprints of new compounds with profiles of reference compounds or those in our substance database. Phenotypic screening is best suited for potential compound repurposing or repositioning projects. With this, it is possible to search for new indications at other compound concentrations or in drug combinations.
This allows:
- Identifying the neuroactive concentration ranges based on multi-parametric concentration-response data (picomolar to micromolar)
- Differentiating your new compounds against target-specific substances, endogenous ligands, and standard-of-care drugs.
- Gaining insight into the functional similarity to reference compounds at specific concentrations (e.g. therapeutic concentration of more than 80 clinical CNS drugs already tested by NeuroProof). This analysis is performed in a concentration-specific manner for the test compound.
- Proposing clinical indication by sorting the profile into several drug classes (e.g. anticonvulsants, antipsychotics, antidepressants, pro-cognitives, analgesics, and more).
- Assess potential repurposing opportunities for your compounds, which might rescue your compounds for which their development was stopped due to safety or efficacy concerns.
- Elucidate potential side effects such as pro-convulsive or sedative (read: prediction of side effects).
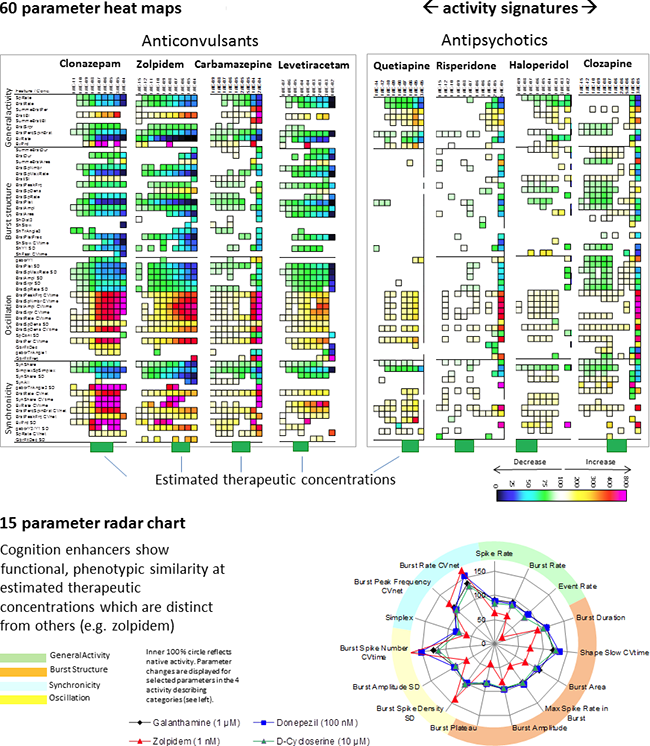
Image: Activity profiled for concentration-response experiments of drugs from our database show obvious functional phenotypic similarities of drugs from one drug class; however, differences between drug classes. Heat maps show significant parameter changes in color codes for every concentration and, thus, represent the compound profile. Estimated therapeutic concentrations were calculated based on literature data on plasma concentration and brain/plasma ratios.
We deliver:
- The phenotypic profiling characterizes a compound's action in the whole concert of all receptors it affects. Our experience shows that each compound exhibits a distinct and quantitatively assessable profile with unique properties.
- A characterization of a compound's action established by our comprehensive multivariate data analysis. You receive a description of your compound's action on neuronal networks with a multitude of parameters (general activity, regularity, burst structure/strength, synchronicity/connectivity/communication).
- An overall comparison to your reference compounds or known compounds of our database.
The following primary cell cultures are available:
- frontal cortex
- hippocampus
- amygdala/hippocampus co-culture
- midbrain
- midbrain/cortex co-culture
- spinal cord
- hypothalamus
These tissue cultures produce highly reproducible activity patterns.
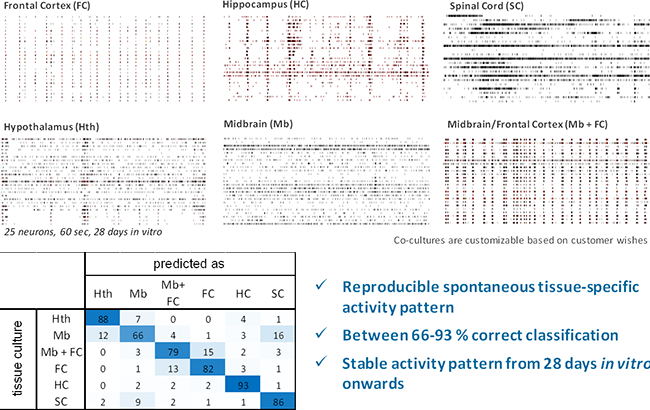
Image: Top, example spike trains from 6 different primary tissue cultures after 28 DIV. Bottom, self-recognition of brain-region-specific activity patterns [% of datasets] from primary cell cultures show that all tissue cultures produce reproducible activity patterns after 28 days in vitro. 4 classification experiments with 100 data sets each.
These tissue cultures from mice are dissected between embryonic days E14 and E18, depending on the tissue. We also establish cell cultures (e.g. co-cultures) based on your needs. Please contact us for more information.
We are developing protocols for human induced pluripotent stem cell-derived neurons from a wide range of cell lines and disease types.

 +49 381 54345-660
+49 381 54345-660
