Fragile X Syndrome Disease Model with iPSC-Derived Neurons
Contact us for further information!
Fragile X syndrome, FXS, is the most common form of inherited cognitive disability. FXS patients also show symptoms that belong to the autism spectrum disorder symptoms. FXS is a neuro-developmental disease with a prevalence of 1:5000 in males and 1:8000 in females.
There is no effective treatment for FXS available at the moment.
NeuroProof is a drug screening company offering new functional phenotypic screening assays for new potential disease-modifying treatments of FXS.
Fragile X Syndrome Disease Etiology
Fragile X syndrome (FXS) is a result of a CGG trinucleotide repeat expansion of the Fragile X mental retardation 1 gene (FMR1), which causes its silencing. In most people, the number of CGG’s repeats range between 5 and 55, in patients, it expands to over 200 or more repeats.
Since copy number variants of the CGG repeats are varying and also the specific genetic background are responsible for high variation in treatment responses.
The technology presented here could also be suitable for patient stratification or precision medicine approaches.
NeuroProof's Functional Phenotypic Screening FXS Model with iPSC Derived Glutamatergic Neurons
NeuroProof uses glutamatergic neurons from a human induced pluripotent stem, iPSC, cell line with an FMR1 mutation, and non-diseased control cell lines. These iPSC-derived glutamatergic neurons are cultivated on microelectrode arrays, MEA, and form spontaneous active neuronal cultures. With this, NeuroProof tests compounds in a functional phenotypic disease model of FXS.
Example of FXS Drug Screening with a Rescue Assay
We used an FXS-patient cell line carrying an FMR1 mutation with 400 copy variants of CGT and control cell lines differentiated into glutamatergic neurons cultivated on 48-well MEA plates. We recorded spike train data at day 20-24 in vitro:

Image: Spike train from iPSC-derived glutamatergic neurons derived from a healthy individual.
![]()
Image: Spike trains from iPSC-derived glutamatergic neurons derived from an individual with a CGG copy variant mutation.
We tested a compound at first that failed in clinical trials. This compound was selected on the base of the E/I balance hypothesis in FXS.
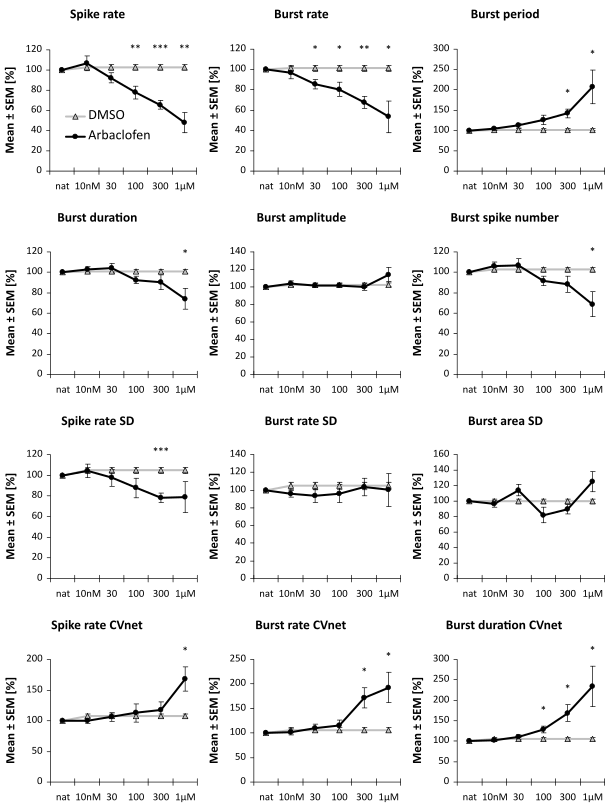
A second compound we tested showed only a mild effect in an acute concentration-response- test, but showed a very similar effect in the effect score at 300 nM, see below.
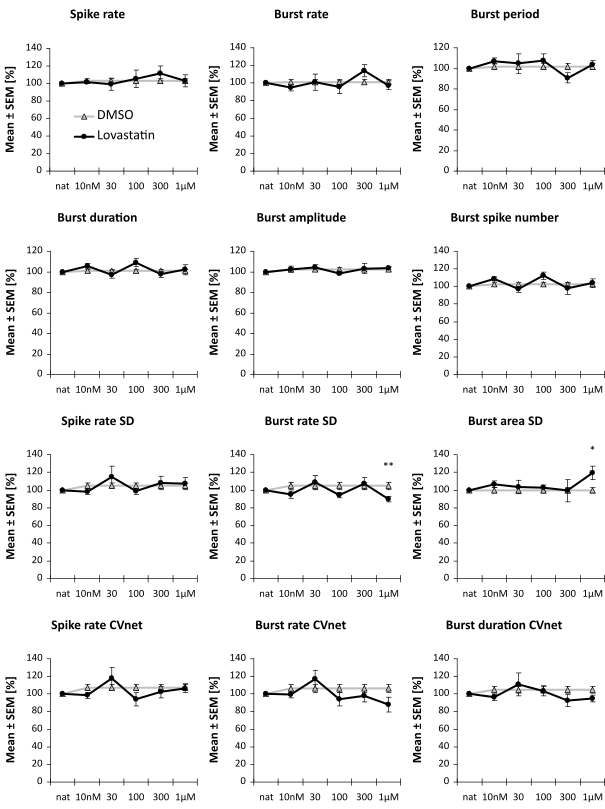
These cell lines were used for a rescue assay. With a specific projection of the spike train parameters into a one-dimensional effect-score, it is possible to assess the putative therapeutic effect of a compound.
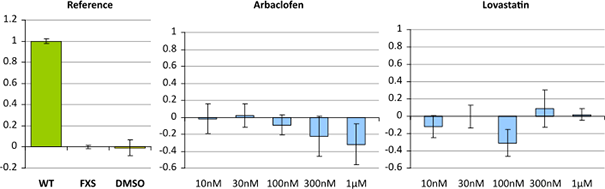
Image: Example of two compounds that have no or only minimal (not significant) effect in an FXS rescue assay. It is assessed the reversion of a compound with the diseased cell line scaled to 1.0 and the healthy cell line scaled to 0.0.
NeuroProof is continuously expanding its assay portfolio, ask us for potential new FXS cell lines also with other iPSC-derived neuronal cell types or potentially positive compounds.

 +49 381 54345-660
+49 381 54345-660
